Carbon Dioxide
There are many chemicals available on the market today that are suitable for use as neutralization chemicals. The most commonly used chemicals are discussed in an article available here: Neutralization Chemicals.
Carbon Dioxide - CO2
Carbon Dioxide (CO2): The third most concentrated gas found in earth’s atmosphere (preceded by nitrogen and oxygen) CO2 is it self not an acid. CO2 forms carbonic acid (H2CO3) when dissolved in water; and it is carbonic acid that leads to the neutralization of alkalinity in solution. Carbon dioxide is not easy to use and its use is limited. However, for some applications CO2 can be a very effective choice. The most appealing feature of CO2 is that it will not lower the pH of water below 7.0 (for practical purposes). Additionally CO2 is not corrosive as a gas, however, since CO2 is heavier than air asphyxiation is always a hazard. Carbon dioxide can be difficult to use because the gas must be dissolved into solution to be used. This requires the use of a carbonator, or some method to dissolve the gas into solution. Generally a tall tank must be used to ensure that there is sufficient fluid pressure to promote the dissolution of CO2 in water. Significant out-gassing will occur, which is not a problem unless the process also requires the settling of solids.
CO2 + H2O ↔ H2CO3
H2CO3 + 2NaOH ↔ Na2CO3 +2H2O
The architecture of a system that uses a gas neutralizing chemical is much different than that which uses a liquid chemical. Therefore the use of CO2 should be limited to unidirectional systems only (i.e. neutralizing alkaline materials only). Bi-directional systems using CO2 require the use of a liquid chemical for the alkaline chemical and a gas for the acidic chemical.
Because of our unique design our pH
adjustment systems can all be configured to use either liquid
mineral acids (such as sulfuric acid) or carbon dioxide. The
efficacy of CO2 as a neutralizing agent should be
confirmed in a laboratory, or through pilot testing, before use.
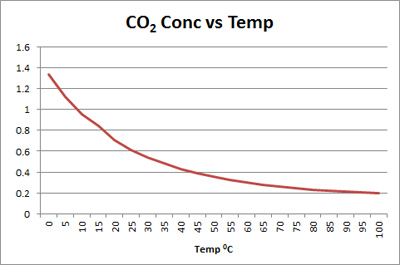 The efficacy of CO2 decreases with elevated temperatures due to decreasing solubility of CO2 in solution as the temperature rises. In most
cases with a temperature above about 600C the use of CO2 may be ill advised and mineral acid may be a better choice.
The efficacy of CO2 decreases with elevated temperatures due to decreasing solubility of CO2 in solution as the temperature rises. In most
cases with a temperature above about 600C the use of CO2 may be ill advised and mineral acid may be a better choice.
Our hydroTREAT family of pH adjustment systems are specifically designed to use CO2 to neutralize alkaline influent wastewater particularly wastewater from concrete runoff, hydro-demolition, and mining applications.
Back to Neutralization Chemicals....
